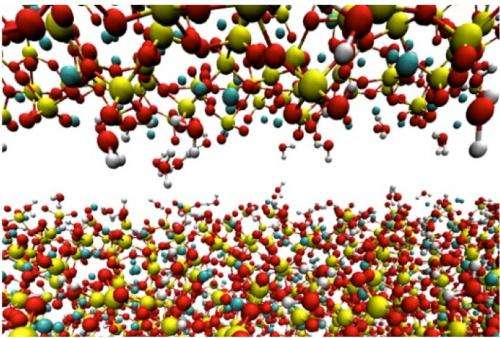
Phys.org reports that the reason concrete is so strong is elemental… according to researchers at Rice University, studying friction on materials such as concrete has provided the answer.
Concrete is considered a “structurally complex” material, with elements in a jumble rather than in an ordered crystalline shape. Because of this, the elements experience a friction at the nanoscale. The Rice researchers undertook detailed studies of this friction and determined that calcium-silicate-hydrate (C-S-H), aka cement, looks like a paste before hardening, but it consists of discrete nanoscale particles. The elemental forces that interplay between the C-S-H and the larger particles in concrete are the key to the material’s overall strength and fracture properties.
















